|
A Critical
Technology
The recent FDA approval of an
electrically powered blood pump to be used as an alternative to
cardiac transplantation will bring into focus a core WBI technology,
methods for transcutaneous energy transmission (TET). Using this technology, no
wires or tubes penetrate the skin to supply power to an implanted blood
pump, and the problem of infection is significantly reduced.
Shown below is
a photograph of a prototype TET system coil assembly. External electronics are
used to convert battery power to a radio frequency that is applied to
the external primary coil. A completely implanted secondary coil receives
this energy and directs it to implanted power conditioning
circuitry that produces a direct current which can be used by
the motor of the blood pump.
The
original TET system was developed by Craig W. Sherman, now Vice President of R&D
at WBI. The outer external primary coil is "worn" on the skin over the
implanted secondary coil. Infection has been the most serious complication associated with blood pumps used
for long periods of support that require percutaneous wires or tubes. TET
preserves the integrity of the skin
as a barrier to bacterial entry.
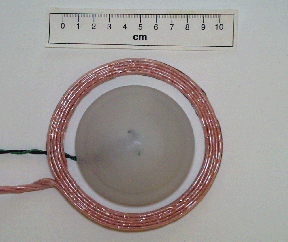
The implanted secondary coil of the TET is made with a
dome shape so that when placed beneath the skin, it provides a simple way of properly align
the coils. A TET secondary is shown below. In this configuration, developed at WBI for the
new rotary blood pumps now entering clinical trials, the TET power
conditioning circuitry is contained within the secondary coil assembly. WBI has developed TET systems suitable for virtually any
implanted blood pump. Currently, we are working with 3 developers of fully implantable
blood pumps to supply TET systems.

|
![]()